The Potential of RIP (Ribosome Inactivating Protein) as Biopesticides
Abstract
Keywords
Full Text:
PDFReferences
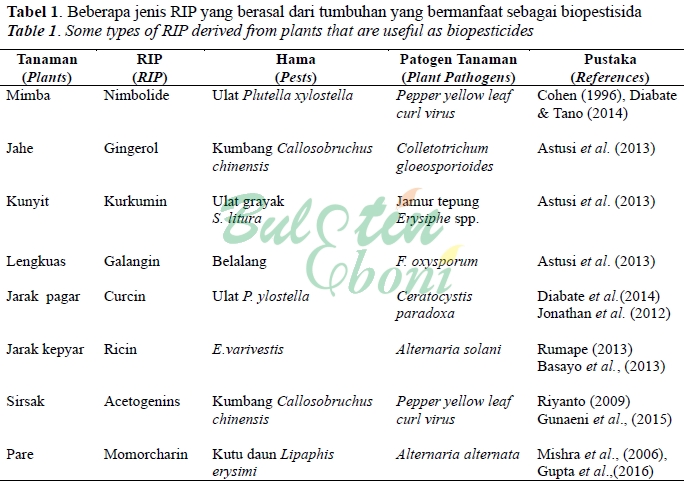
Astuti, U.P. T. Wahyuni dan B. Honorita. (2013). Petunjuk Teknis Pembuatan Pestisida Nabati. Balai Pengkajian Teknologi Pertanian Bengkulu. 70 p.
Astuthi, M.M.M., K. Sumiartha, I.W. Susila, G. N. A. S. Wirya dan I.P. Sudiarta. (2012). Efikasi minyak atsiri tanaman cengkeh (Syzygium aromaticum (L.) Meer. & Perry), pala (Myristica fragrans Houtt), dan jahe (Zingiber officinale Rosc.) terhadap mortalitas ulat bulu gempinis dari famili Lymantriidae. Journal of Agricultural Science and Biotechnology, 1(1), 14-23.
Basayo, I., H. Nahunnaro and D. M. Gwary. (2013). Effect of aqueous exreact of Ricinus communis on radial growth of Alternaria solani. African Journal of Agricultural Research, 8(36), 4541-4545.
Bozza, W.P., W. H. Tolleson, L. A. R. Rosado and B. Zhang. (2015). Ricin detection: tracking active toxin. Biotechnology Advances, 33(1), 117-123.
Choudhary, N., H. C. Kapoor and M. L. Lodha. (2008). Cloning and expression of antiviral/ribosome-inactivating protein from Bougainvillea xbuttiana. Journal of Biosciences, 33(1), 91-101.
Citores, L., R. Iglesias, C. Gay and J. M. Ferreras. (2016). Antifungal activity of the ribosome-inactivating protein BE27 from sugar beet (Beta vulgaris L.) against the green mould Penicillium digitatum. Molecular Plant Pathology, 17(2), 261-271.
Cohen, E., G.B. Quistad andJ.E. Casida. (1996). Cytotoxicity of nimbolide, epoxyazadiradione and other limonoids from neem insecticide. Life Sciences, 58(13), 1075-1081.
Diabate, D., J.A. Gnago & Y. Tano. (2014). Toxicity, antifeedant and repellent effect of Azadirachta indica(A. Juss) and Jatropha carcusL. aqueous extracts against Plutella xylostella(Lepidoptera: Plutellidae). Journal of Basic and Applied Scientific Research, 4(11), 51-60.
Djunaedi, A. 2009. Biopestsida sebagai pengendali organisme pengganggu tanaman (OPT) yang ramah lingkungan. Embryo, 6(1), 88-95.
Faraq, H. R., Z. A. Abdou, D. A. salama, M. Ibrahim and H. Srour. (2011). Effect of neem and willow aqueous extracts on fusarium wilt disease in tomato seedlings: inducton of antoxidant defensive enzymes. Annals of Agricultural Sciences, 56(1), 1-7.
Fuseini, Z. (2010). Occurrence and control of seedborne pathogenic fungi of tomato (Lycopersicon esculentum Mill.) seeds from five agro-ecological zones of Ghana using plant extracts. [Thesis]. Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
Grossi de Sa, M.F., P. B. Pelegrini, I. M. Vasconcelos, C. R. Carlini and M. S. Silva. (2015). Entomotoxic plant proteins: potential molecules to develop genetically modified plants resistant to insect-pest. Plant Toxins, Toxinology,34 p.
Gunaeni, N., A.W. Wulandari dan A. Hudayya. (2015). Pengaruh bahan ekstrak tanaman terhadap pathogenesis related proteindan asam salisilat dalam menginduksi resistensi tanaman cabai merah terhadap virus kuning keriting. Jurnal Hortikultura, 25(2), 160-170.
Gupta, M. S. Sharma and R. Bhadauria. (2017). Phytotoxicity of Momordica charantia extracts against Alternaria alternata. J. Pharm. Sci. & Res., 9(1), 28-34.
Hayati, R., T. Chamzurni dan B. Amin. (2016). Aplikasi beberapa fungisida nabati dengan berbagai dosis untuk mengendalikan penyakit layu Fusarium (Fusarium oxysporum) pada tanaman tomat. Jurnal Ilmiah Mahasiswa Pertanian Unsyiah, 1(1), 261-269.
Hedge, Y.R. andR.S.Keshgond. (2013). Role of pathogenesis-related proteins in plant disease management – a review. Agricultural Reviews 34(2), 145-151.
Islam,M. T. and A.N. Faruq. (2012). Effect of some medicinal plant extracts on damping-off disease of winter vegetable. World Applied Sciences Journal, 17(11), 1498-1503.
Isnaini, M., E. R. Pane dan S. Wiridianti. (2015). Pengujian beberapa jenis insektisida nabati terhadap kutu beras (Sitophilus oryzae L.). Jurnal Biota, 1(1), 1-8.
Jonathan, S. G., E. M. Udoh, E. E. Ojome, O. J. Olawuyi and B. J. Babalola. (2012). Efficacy of Jatropha curcas Linn. As fungicides in the control of Ceratocystis paradoxa (chalara anamorph) IMI 501775 associated with bole rot of Cocos nucifera Linn. Seedlings. Report and Opinion, 4(12), 48-60.
Khasanah, A. R. (2011). Pemanfaatan minyak biji jarak pagar (Jatropha curcas L.) sebagai insektisida nabati terhadap mortalitas larva Spodoptera litura F. (ulat grayak). [Skripsi). Jurusan Biologi, Fakultas Sains dan Teknologi, Universitas Islam Negeri Maulana Malik Ibrahim, Malang.
Kodjo, T. A., M. Gbenonchi, A. Sadate, A. Komi, G. Y. M. Dieudonne and S. K. E. Cole. (2011). Bio-insecticidal effects of plant extracts and oil emulsions of Ricinus communis L. (Malpighiales: Euphorbiaceae) on the diamondback, Plutella xylostella L. (Lepidoptera: Plutellidae) under laboratory and semi-field conditions. Journal of Applied Biosciences, 43, 2899-2914.
Lafontaine, D. L. J. and D. Tollerve. (2001). Ribosomal RNA. Encyclopedia Life Sciences.
Mishra, D., A.K.Shukla, A.K. Dubey, A.K.Dixit and K.Singh. (2006). Insecticidal activity of vegetable oils against mustard aphid,Lipaphis erysimiKalt., under field condition. Journal of Oleo Science, 55(5), 227-231.
Mondal, N. K., A. Mojumdar, S. K. Chatterje, A. Banerjee, J. K. Datta, S.Gupta. (2009). Antifungal activities and chemical characterization of neem leaf extracts on the growth of some selected fungal species in vitro culture medium. Appl. Sci. Envron. Manage., 13 (1), 49-53.
Mossini, A. A. G., C. C. Arroteia and C. Kemmelmeier. (2009). Effect of neem leaf extract and neem oil on Penicillium growth, sporulation, morphology and Ochratoxin A production.Toxins, 1, 3-13.
Nahak, G and R. K. sahu. (2014). Bioeffcacy of leaf extracts of neem (Azadirachta indica A. Juss.) on growth parameters, wilt and leaf spot diseases of Brinjal. Research Journal of Medicinal Plant, 8 (6), 269-276.
Narayanan, S., N. Bora, A. Surolia & A.A. Karande. (2005). Ribosome inactivating proteins and apoptosis. FEBS Letters, 579, 1324–1331.
Park, S. W., B. Prithiviraj, R. Vepachedu and J. M. Vivanco. (2006). Methods in Molecular Biology: isolation and purification of Ribosome-Inactivating Proteins in Plant Cell Culture Protocols (Loyola-Vargas, V.M and Vazquez-Flota F.Eds.) 318:335-347. Humana Press. New Jersey.
Rahayuningtias, S. dan W. S. Harijani. (2017). Kemampuan pestisida nabati (mimba, gadung, laos dan serai), terhadap hama tanaman kubis (Brassica Oleracea L.).Agritop, 15(1), 207-211.
Riyanto. (2009). Potensi lengkuas (Languas galangal L.), beluntas (Pluchea indica L.), dan sirsak (Annona muricata L.) sebagai insektisida nabati kumbang kacang hijau Callosobruchus chinensis L. (Coleoptera: Bruchidae). Sainmatika, 6(2), 58-66.
Rukmi, I. (2019). Kenakeragaman Aspergillus pada berbagai simplisia jamur tradisional. Jurnal Sains & Matematika, 17(2), 82-89.
Rumape, O. (2013). Isolasi dan identifikasi senyawa antifeedant dari daun jarak kepyar (Ricinus communis L.) terhadap kumbang Epilachna varivestis Mulsant. [Disertasi]. Universitas Negeri Gorontalo.
Saxena, R. C. (2015). Mimba untuk Pengendalian Hama dan Konservasi Lingkungan yang Berkelanjutan [Terjemahan]. ECHO Asa Notes 24: 27p.
Sharma, N, S. W. Park, R. Vepachedu, L. Barbieri, M. Ciani, F. Stirpe, B. J. Savary and J. M. Vivanco. (2004). Isolation and characterization of an rip (ribosomeinactivating protein)-like protein from tobacco with dual enzymatic activity. Plant Physiology, 134, 171–181.
Starngalin, J. R., O. J. Kuhn, L. Assi and K. R. F. Schwan-Estrada. (2011). Control of plant diseases using extracts from medicinal plants and fungi. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances. A. Mendez-Vilas (Ed.). Formatex.
Stirpe, F. and R. G. Oriol. (2015). rRbosome-inactivating proteins: an overview. Plant Toxins, Toxinology, 27 p.
Sumartini dan E. Yusnawan. (2005). Pengaruh jenis dan konsentrasi bahan nabati terhadap perkembangan Aspergillus flavus pada medium PDA dan biji kacang tanah. Majalah Ilmiah Biologi BIOSFERA, 22(1), 25-29.
Vivanco, J.M., B.J. Savary dan H.E. Flores. (1999). Characterization of two novel type I ribosome-inactivating proteins from the storage roots of the Andean Crop Mirabilis expansa. Plant Physiology, 119, 1447–1456.
Wang P. dan N. E. Tumer. (1999). Pokeweed antiviral protein cleaves double-stranded supercoiled DNA using the same active site required to depurinate rRNA. Nucleic Acids Research, 27(8), 1900-1905
Wiratno, Siswanto, Luluk dan S. Suriati. (2016). Efektivitas jenis tanaman obat dan aromatik sebagai insektisda nabati untuk mengendalikan Diconocoris hewtti Dst (Hemiptera: Tingidae). Bul. Littro., 22(2), 198-204.
Yadav S., A. David, F. Baluska, S.C. Bhatla. (2013). Rapid auxin-induced nitric oxide accumulation and subsequent tyrosine nitration of proteins during adventitious root formation in sunflower hypocotyls. Plant Signaling & Behaviour, 8(3) e23196.
DOI: https://doi.org/10.20886/buleboni.5271
Refbacks
- There are currently no refbacks.
Copyright (c) 2019 Buletin Eboni

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Indexed by:
 BULETIN EBONI
BULETIN EBONI



 buletin_eboni@balithutmakassar.org
buletin_eboni@balithutmakassar.org